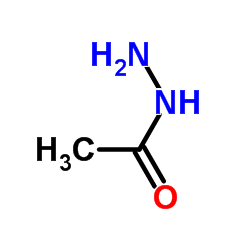
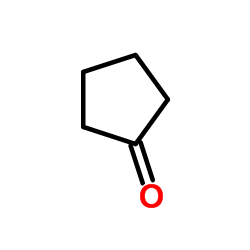
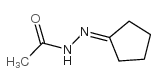
|
~% |
|
~65% |
|
~% |
|
~% |
|
~54% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~74% |



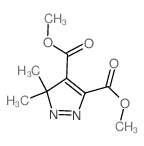
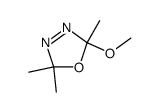
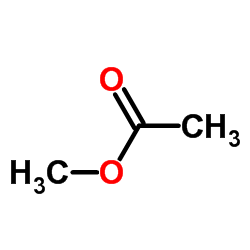
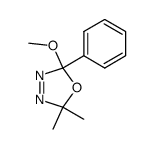
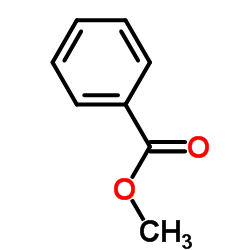
![4-Oxa-1,2-diazaspiro[4.4]non-1-ene,3-methoxy-3-methyl-(9CI)结构式](https://image.chemsrc.com/caspic/444/119393-19-0.png)
![dimethyl 1,2-diazaspiro[4.4]nona-1,3-diene-3,4-dicarboxylate结构式](https://image.chemsrc.com/caspic/313/119393-27-0.png)

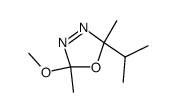

![1,2-Diazaspiro[4.5]deca-1,3-dien-3,4-dicarbonsaeure-dimethylester结构式](https://image.chemsrc.com/caspic/277/82942-51-6.png)