| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
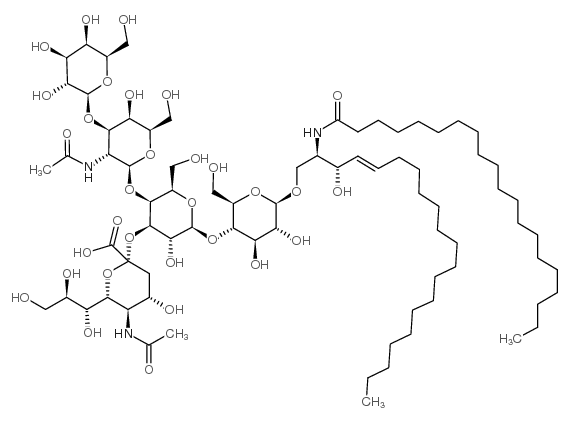 |
GM1 神经节苷脂钠盐,来源于猪脑
CAS:37758-47-7 |
|
 |
霍乱毒素
CAS:9012-63-9 |