| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
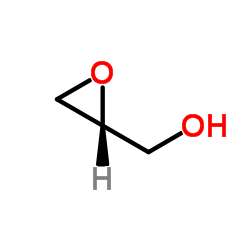 |
(|R|)-(+)-缩水甘油
CAS:57044-25-4 |
|
 |
缩水甘油
CAS:556-52-5 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
(|R|)-(+)-缩水甘油
CAS:57044-25-4 |
|
 |
缩水甘油
CAS:556-52-5 |