Nucleophilic substitution at the anomeric position of 1,2-O-isopropylidenefuranose derivatives. A novel stereoselective synthesis of cyclic phosphates analogous to cAMP.
Miriam Romero, Luís Hernández, Leticia Quintero, Fernando Sartillo-Piscil
文献索引:Carbohydr. Res. 341(18) , 2883-90, (2006)
全文:HTML全文
摘要
1,2-O-Isopropylidenefuranose derivatives were treated with various nucleophiles in the presence of either BF(3).OEt(2) or trimethylsilyl trifluoromethanesulfonate (TMSOTf) leading to substitution products in a regio- and stereoselective manner. In particular, nucleophilic substitution of 1,2-O-isopropylidenefuranose derivatives when treated with allyltrimethylsilane was controlled by steric and electronic factors (similar to Woerpel's stereoelectronic model). On the other hand, when 1,2-O-isopropylidenefuranose derivatives were treated with trimethylsilane, in the presence of bis-O-trimethylsilyl-5-iodouracil or bis-O-trimethylsilyl-thymidine, substitution products were generated in high regio- and stereoselectivities via an unusual nucleophilic substitution with opening of the furanose ring. Based on these results, a stereoselective method for the synthesis of neutral cyclic phosphates analogous to cAMP was developed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
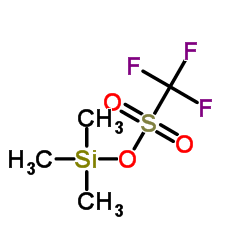 |
三氟甲磺酸三甲基硅酯
CAS:27607-77-8 |
C4H9F3O3SSi |
|
Trimethylsilyl trifluoromethanesulfonate-promoted reductive ...
2011-06-01 [Nucleosides Nucleotides Nucleic Acids 30(6) , 446-56, (2011)] |
|
Trimethylsilyl trifluoromethanesulfonate (TMSOTf) assisted f...
2008-01-18 [J. Org. Chem. 73(2) , 752-5, (2008)] |
|
Intramolecular formal [4 + 2] cycloaddition of nitriles with...
2009-08-07 [J. Org. Chem. 74(15) , 5699-702, (2009)] |
|
Diastereoselective synthesis of substituted dihydropyrans vi...
2012-11-21 [Org. Biomol. Chem. 10(43) , 8730-8, (2012)] |
|
Facile synthesis of N-(1-alkenyl) derivatives of 2,4-pyrimid...
2000-07-01 [Nucleosides Nucleotides Nucleic Acids 19 , 1093-1100, (2000)] |