| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
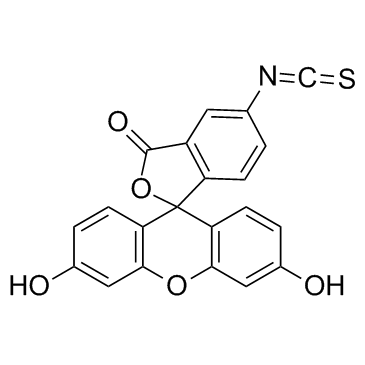 |
异硫氰酸荧光素酯
CAS:3326-32-7 |
|
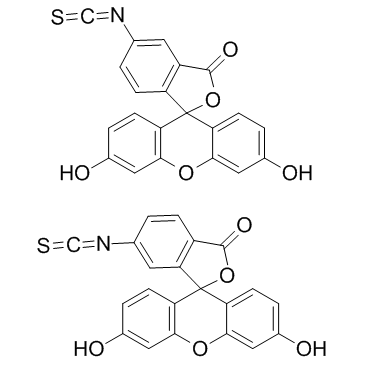 |
异硫氰酸荧光素
CAS:27072-45-3 |
|
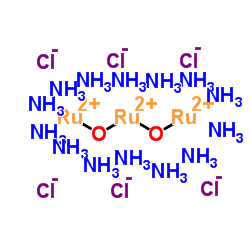 |
钌红
CAS:11103-72-3 |
|
![5-[4-苯基-5-(三氟甲基)-2-噻吩基]-3-[3-(三氟甲基)苯基]-1,2,4-恶二唑 结构式](https://image.chemsrc.com/caspic/396/256414-75-2.png) |
5-[4-苯基-5-(三氟甲基)-2-噻吩基]-3-[3-(三氟甲基)苯基]-1,2,4-恶二唑
CAS:256414-75-2 |
|
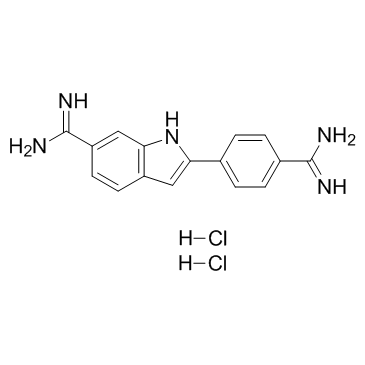 |
4',6-二脒基-2-苯基吲哚二盐酸盐
CAS:28718-90-3 |
|
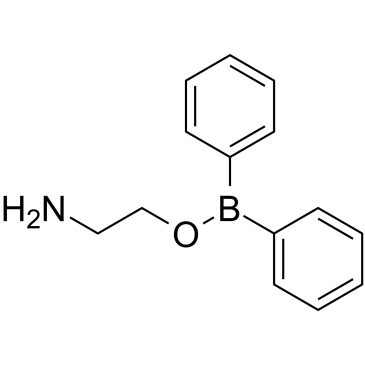 |
2-氨基乙基联苯基硼酸酯
CAS:524-95-8 |