| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
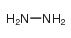 |
水合肼
CAS:10217-52-4 |
|
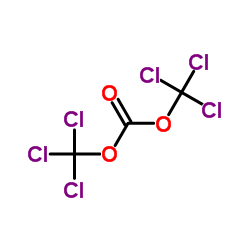 |
三光气
CAS:32315-10-9 |
|
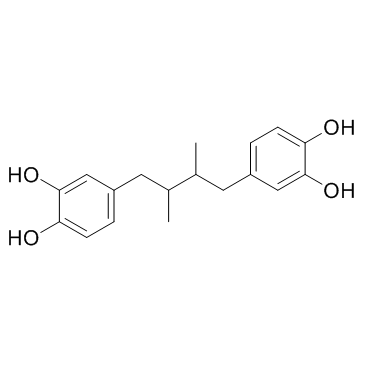 |
去甲二氢愈创木酸
CAS:500-38-9 |
|
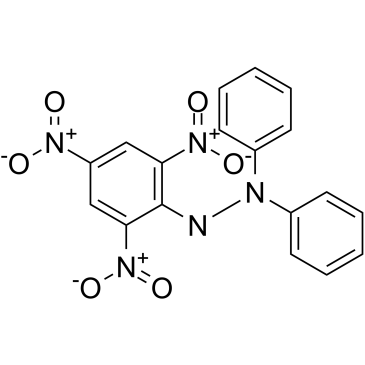 |
2,2-联苯基-1-苦基肼基
CAS:1898-66-4 |
|
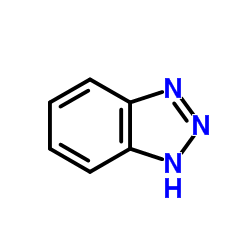 |
苯并三氮唑
CAS:95-14-7 |
|
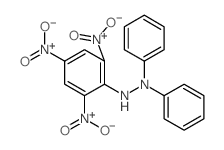 |
1,1-二苯基-2-苦味酰肼
CAS:1707-75-1 |
|
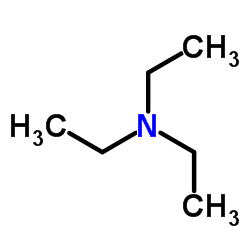 |
三乙胺
CAS:121-44-8 |
|
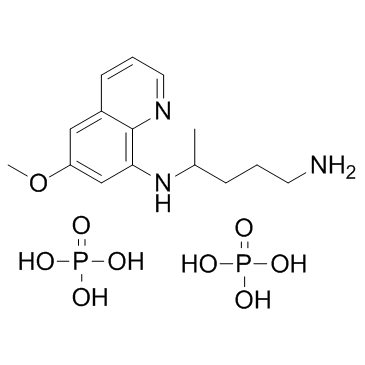 |
磷酸伯安喹
CAS:63-45-6 |
|
 |
N-三苯甲基-1,2-乙二胺 氢溴酸盐
CAS:389064-43-1 |