| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
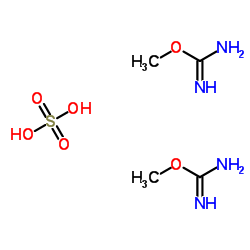 |
O-甲基异脲硫酸盐
CAS:52328-05-9 |
|
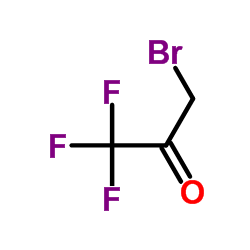 |
3-溴-1,1,1-三氟丙酮
CAS:431-35-6 |
|
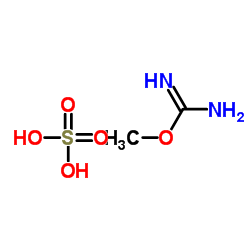 |
O-甲基异脲硫酸盐
CAS:29427-58-5 |