Effect of a covalently attached synergistic anion on chelator-mediated iron-release from ovotransferrin: additional evidence for two concurrent pathways.
C T Bailey, C Byrne, K Chrispell, C Molkenbur, M Sackett, K Reid, K McCollum, D Vibbard, R Catelli
文献索引:Biochemistry 36(33) , 10105-8, (1997)
全文:HTML全文
摘要
The mechanism by which the iron-transport protein transferrin releases its iron in vivo is presently unclear. In vitro studies have implicated two concurrent chelator-mediated iron-release pathways: one which is hyperbolic in nature, involving a conformational change in the protein as a rate limiting step, and a second which has been proposed to be first-order in nature and to involve initial release of a synergistic anion. We have examined the effect that an affinity-label analog of the synergistic anion has on chelator-mediated iron-release from this protein. A covalently attached anion would inhibit iron-release via any pathway in which anion release is a prerequisite to iron release. The present investigation examined the effect that the covalently attached anion had on iron-release to pyrophosphate (PPi) and N, N-bis(phosphonomethyl)glycine (DPG), two chelators which are believed to utilize both pathways concurrently. Results show that when the affinity-label anion is utilized, strictly hyperbolic data are obtained, with similar observed kmax values. This is strong support for the hypothesis of a common, chelator-independent rate-limiting step for the one available pathway. These results also support strongly the hypothesis that synergistic anion removal is a prerequisite step to iron-release via the second pathway.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
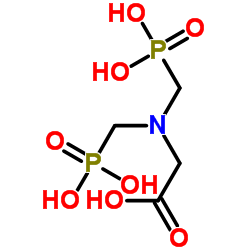 |
增甘磷
CAS:2439-99-8 |
C4H11NO8P2 |
|
Structure-based selection of small molecules to alter allele...
2011-12-01 [J. Immunol. 11th ed., 187 , 5921-5930, (2011)] |
|
Binding of phosphonate chelating agents and pyrophosphate to...
1991-07-16 [Biochemistry 30(28) , 6930-6, (1991)] |
|
Synthesis, characterization, and in vitro antitumor activity...
2003-11-06 [J. Med. Chem. 46(23) , 4946-51, (2003)] |
|
Influence of feeding chlorocholine chloride and glyphosine o...
1984-03-01 [Toxicology 30(2) , 103-14, (1984)] |
|
Rapid and sensitive determination of phosphorus-containing a...
2005-12-01 [Electrophoresis 26(23) , 4478-85, (2005)] |