| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
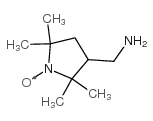 |
3-(氨基甲基)-2,2,5,5-四甲基-1-吡咯烷氧基
CAS:54606-49-4 |
|
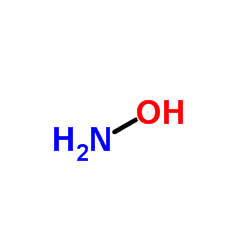 |
羟胺
CAS:7803-49-8 |
|
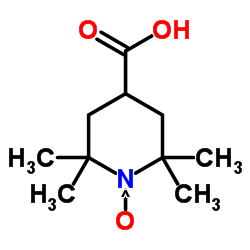 |
4-羧基-2,2,6,6-四甲基氮杂环己烷-1-氧基自由基
CAS:37149-18-1 |