| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
锌标准溶液
CAS:7440-66-6 |
|
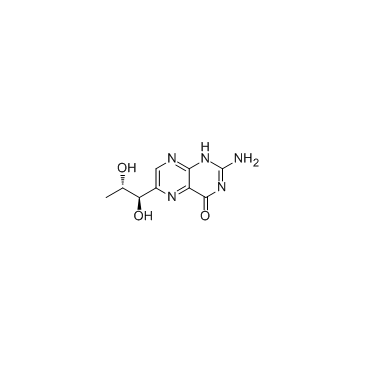 |
L-生物喋呤
CAS:22150-76-1 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
锌标准溶液
CAS:7440-66-6 |
|
 |
L-生物喋呤
CAS:22150-76-1 |