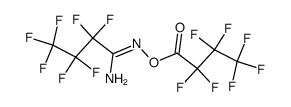
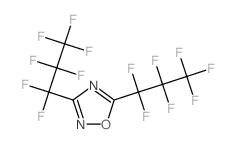
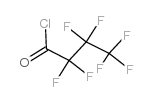
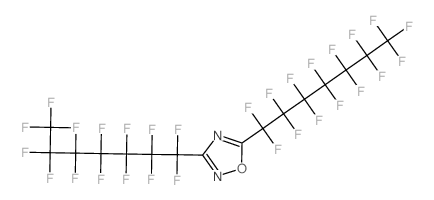
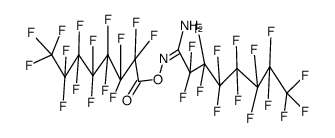
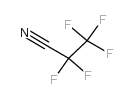
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
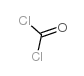

![[(1-amino-2,2,3,3,4,4,4-heptafluoro-butylidene)amino] chloroformate结构式](https://image.chemsrc.com/caspic/149/4368-74-5.png)
![[(1-amino-2,2,3,3,3-pentafluoro-propylidene)amino] 2,2,3,3,3-pentafluoropropanoate结构式](https://image.chemsrc.com/caspic/214/4396-71-8.png)