| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
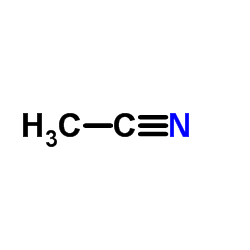 |
乙腈
CAS:75-05-8 |
|
 |
甲醇
CAS:67-56-1 |
|
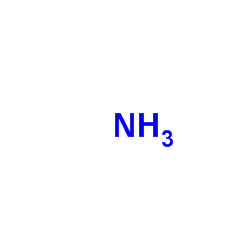 |
氨
CAS:7664-41-7 |
|
 |
AMMONIA (14N)
CAS:1026405-88-8 |
|
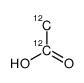 |
乙酸-12C2
CAS:1173022-32-6 |
|
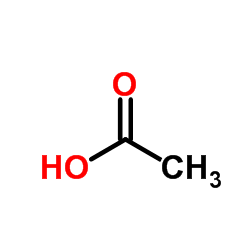 |
冰醋酸
CAS:64-19-7 |
|
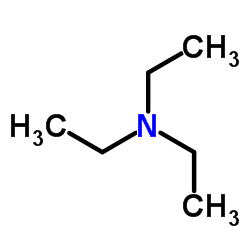 |
三乙胺
CAS:121-44-8 |
|
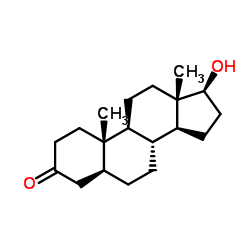 |
雄诺龙
CAS:521-18-6 |
|
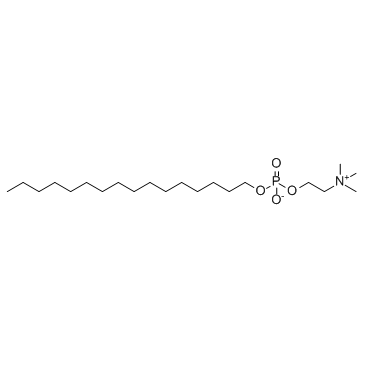 |
米替福新
CAS:58066-85-6 |