| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
乙腈
CAS:75-05-8 |
|
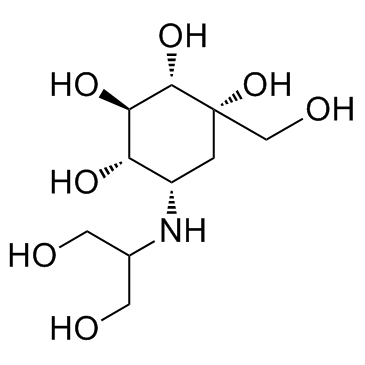 |
伏格列波糖
CAS:83480-29-9 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
乙腈
CAS:75-05-8 |
|
 |
伏格列波糖
CAS:83480-29-9 |