Metabolism of echinacoside, a good antioxidant, in rats: isolation and identification of its biliary metabolites.
Cunqin Jia, Haiming Shi, Wei Jin, Ke Zhang, Yong Jiang, Mingbo Zhao, Pengfei Tu
文献索引:Drug Metab. Dispos. 37(2) , 431-8, (2009)
全文:HTML全文
摘要
Echinacoside (ECH) is one of the major active phenylethanoid glycosides (PEGs) in famous traditional Chinese medicine, Herba Cistanches. Although it has various bioactivities, such as antioxidation, neuroprotection, and hepatoprotection, knowledge about its metabolic fate is scant. In the present study, eight phase II metabolites, 3,4 -O-dimethyl-ECH-3 -O-beta-d-glucuronide (M1); 4,4 -O-dimethyl-ECH-3 -O-beta-d-glucuronide (M2); 3,4 -O-dimethyl-ECH-4-O-sulfate ester (M3); 4,4 -O-dimethyl-ECH-3-O-sulfate ester (M4); 3,3 -O-dimethyl-ECH (M5); 3,4 -O-dimethyl-ECH (M6); 4,3 -O-dimethyl-ECH (M7); and 4,4 -O-dimethyl-ECH (M8), were isolated from rat bile sample after intravenous administration of ECH and identified by mass spectra and NMR spectroscopy, including (1)H NMR, (13)C NMR, nuclear Overhauser effect difference spectroscopy, and two-dimensional NMR (heteronuclear single quantum correlation, heteronuclear multiple-bond correlation spectroscopy, gradient-selected correlation spectroscopy, and nuclear Overhauser effect spectroscopy). Among them, M5 to M8 were O-di-methylated conjugates; M1 and M2 and M3 and M4 were O-dimethyl glucuronides and O-dimethyl sulfates, respectively. In the three types of metabolites of rat, the major metabolites were the methyl ethers and the glucuronides, whereas the sulfates were minor. The regioselectivity of conjugation for ECH and metabolic pathway of ECH were proposed, which gave insight into the mechanism of ECH for its bioactivities in vivo.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
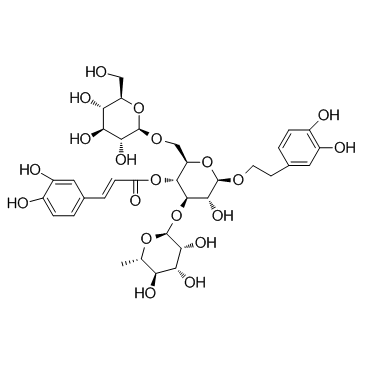 |
松果菊苷
CAS:82854-37-3 |
C35H46O20 |
|
Plantago lanceolata L. water extract induces transition of f...
2015-01-01 [J. Pharm. Pharmacol. 67(1) , 117-25, (2014)] |
|
Echinacoside Induces Apoptosis in Human SW480 Colorectal Can...
2015-01-01 [Int. J. Mol. Sci. 16 , 14655-68, (2015)] |
|
Echinacoside induces apoptotic cancer cell death by inhibiti...
2015-01-01 [Onco. Targets Ther. 8 , 3649-64, (2015)] |
|
Production of acteoside from Cistanche tubulosa by β-glucosi...
2011-04-01 [Pak. J. Pharm. Sci. 24(2) , 135-41, (2011)] |
|
Antiosteoporotic activity of echinacoside in ovariectomized ...
2013-04-15 [Phytomedicine 20(6) , 549-57, (2013)] |