Structural analysis of the interaction between Shiga toxin B subunits and linear polymers bearing clustered globotriose residues.
Miho Watanabe, Katsura Igai, Koji Matsuoka, Atsushi Miyagawa, Toshiyuki Watanabe, Ryohei Yanoshita, Yuji Samejima, Daiyo Terunuma, Yasuhiro Natori, Kiyotaka Nishikawa
文献索引:Infect. Immun. 74(3) , 1984-8, (2006)
全文:HTML全文
摘要
We previously developed linear polymers bearing clustered trisaccharides of globotriaosylceramide (Gb3) as orally applicable Shiga toxin (Stx) neutralizers. Here, using a Gb3 polymer with a short spacer tethering the trisaccharide to the core, we found that shortening the spacer length markedly reduced the binding affinity for Stx2 but not Stx1. Moreover, mutational analysis revealed that the essential binding sites of the terminal trisaccharides were completely different between Stx1 and Stx2. These results provide the molecular basis for the interaction between Stx B subunits and Gb3 polymers.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
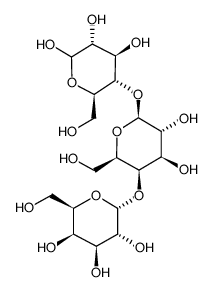 |
Globotriose
CAS:66580-68-5 |
C18H32O16 |
|
Carbosilane dendrimers bearing globotriaoses: syntheses of g...
2006-08-01 [Biomacromolecules 7(8) , 2274-83, (2006)] |
|
Efficient chemoenzymatic synthesis of globotriose and its de...
2002-06-05 [Carbohydr. Res. 337(11) , 969-76, (2002)] |
|
Determination of the cell adhesion specificity of Streptococ...
1999-01-01 [Glycoconj. J. 16(1) , 67-71, (1999)] |
|
Structure of extended lipopolysaccharide glycoforms containi...
2003-04-22 [Biochemistry 42(15) , 4463-75, (2003)] |
|
In vitro and in vivo effects of soluble, monovalent globotri...
2005-09-01 [Antimicrob. Agents Chemother. 49(9) , 3842-6, (2005)] |