Cell-associated collagenolytic activity by Candida albicans.
Masahiro Nishimura, Hiroki Nikawa, Hirofumi Yamashiro, Haruki Nishimura, Taizo Hamada, Graham Embery
文献索引:Mycopathologia 153(3) , 125-8, (2002)
全文:HTML全文
摘要
Cell associated collagenolytic activity of Candida albicans was quantified by measuring the degradation of synthetic peptide 2-furanacryloyl-Leu-Gly-Pro-Ala (FALGPA), which is a specific substrate for collagenase, by the freeze-thaw procedure method. This collagenolytic activity was enhanced by cells cultured in the presence of bovine serum albumin (BSA) in culture medium. However, this activity was inhibited in the presence of ethylenediaminetetraacetic acid disodium salt (EDTA-2Na), but not by the serine proteinase inhibitor p-amidinophenyl methanesulfonyl fluoride (APMSF), nor the aspartyl proteinase inhibitor pepstatin A. These results suggested the presence of a metalloenzyme on pericellular C. albicans.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
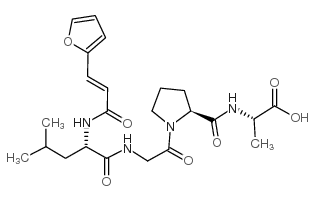 |
胶原酶
CAS:78832-65-2 |
C23H32N4O7 |
|
Aeromonas piscicola AH-3 expresses an extracellular collagen...
2015-03-01 [Lett. Appl. Microbiol. 60(3) , 288-97, (2015)] |
|
University of Wisconsin solution inhibits the class II colla...
1995-12-01 [Transplant. Proc. 27(6) , 3286, (1995)] |
|
Characterization of PepB, a group B streptococcal oligopepti...
1996-08-01 [Infect. Immun. 64(8) , 3401-6, (1996)] |
|
A continuous spectrophotometric assay for Clostridium histol...
1981-05-15 [Anal. Biochem. 113 , 356, (1981)] |
|
Cell-associated collagenolytic activity by group B streptoco...
1994-12-01 [Infect. Immun. 62(12) , 5647-51, (1994)] |