Synthesis, Spectroscopic Characterization, and In Vitro Antimicrobial Studies of Pyridine-2-Carboxylic Acid N'-(4-Chloro-Benzoyl)-Hydrazide and Its Co(II), Ni(II), and Cu(II) Complexes.
Jagvir Singh, Prashant Singh
文献索引:Bioinorg. Chem. Appl. 2012 , 104549, (2012)
全文:HTML全文
摘要
N-substituted pyridine hydrazide (pyridine-2-carbonyl chloride and 4-chloro-benzoic acid hydrazide) undergoes hydrazide formation of the iminic carbon nitrogen double bond through its reaction with cobalt(II), nickel(II), and copper(II) metal salts in ethanol which are reported and characterized based on elemental analyses, IR, solid reflectance, magnetic moment, molar conductance, and thermal analysis (TG). From the elemental analyses data, 1 : 2 metal complexes are formed having the general formulae [MCl(2)(HL)(2)] ·yH(2)O (where M = Co(II), Ni(II), and Cu(II), y = 1-3). The important infrared (IR) spectral bands corresponding to the active groups in the ligand and the solid complexes under investigation were studied. IR spectra show that ligand is coordinated to the metal ions in a neutral bidentate manner with ON donor sites. The solid complexes have been synthesized and studied by thermogravimetric analysis. All the metal chelates are found to be nonelectrolytes. From the magnetic and solid reflectance spectra, the complexes (cobalt(II), nickel(II), and copper(II)) have octahedral and square planner geometry, respectively. The antibacterial and antifungal activity's data show that the metal complexes have a promising biological activity comparable with the parent ligand against bacterial and fungal species.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
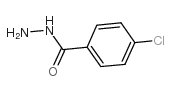 |
4-氯苯甲酰肼
CAS:536-40-3 |
C7H7ClN2O |
|
Eco-friendly and efficient one-pot synthesis of alkyl- or ar...
2008-01-01 [Bioorg. Med. Chem. Lett. 18(1) , 436-8, (2008)] |
|
Bent-shaped mesogenic oxadiazole and thiadiazole derivatives...
[Liq. Cryst. 37(4) , 407-415, (2010)] |
|
Synthesis of benzaldehyde substituted phenyl carbonyl hydraz...
[Int. J. PharmTech Res. 1(4) , 1605-1611, (2009)] |