| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
镁标准溶液
CAS:7439-95-4 |
|
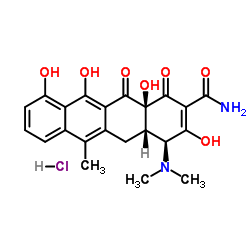 |
盐酸脱水四环素
CAS:13803-65-1 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
镁标准溶液
CAS:7439-95-4 |
|
 |
盐酸脱水四环素
CAS:13803-65-1 |