| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
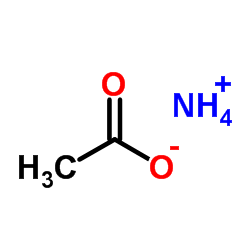 |
乙酸铵
CAS:631-61-8 |
|
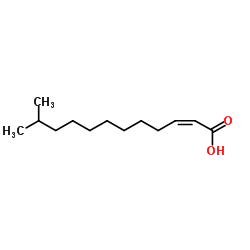 |
顺式-11-甲基-2-十二碳烯酸
CAS:677354-23-3 |
|
 |
麦芽糖
CAS:69-79-4 |