Investigation of thiol-ene and thiol-ene-methacrylate based resins as dental restorative materials.
Neil B Cramer, Charles L Couch, Kathleen M Schreck, Jacquelyn A Carioscia, Jordan E Boulden, Jeffrey W Stansbury, Christopher N Bowman
文献索引:Dent. Mater. 26(1) , 21-8, (2010)
全文:HTML全文
摘要
The objective of this work was to evaluate thiol-norbornene and thiol-ene-methacrylate systems as the resin phase of dental restorative materials and demonstrate their superior performance as compared to dimethacrylate materials.Polymerization kinetics and overall functional group conversions were determined by Fourier transform infrared spectroscopy (FTIR). Flexural strength and modulus were determined with a 3-point flexural test. Polymerization-induced shrinkage stress was measured with a tensometer.Thiol-ene polymer systems were demonstrated to exhibit advantageous properties for dental restorative materials in regards to rapid curing kinetics, high conversion, and low shrinkage and stress. However, both the thiol-norbornene and thiol-allyl ether systems studied here exhibit significant reductions in flexural strength and modulus relative to BisGMA/TEGDMA. By utilizing the thiol-ene component as the reactive diluent in dimethacrylate systems, high flexural modulus and strength are achieved while dramatically reducing the polymerization shrinkage stress. The methacrylate-thiol-allyl ether and methacrylate-thiol-norbornene systems both exhibited equivalent flexural modulus (2.1+/-0.1 GPa) and slightly reduced flexural strength (95+/-1 and 101+/-3 MPa, respectively) relative to BisGMA/TEGDMA (flexural modulus; 2.2+0.1 GPa and flexural strength; 112+/-3 MPa). Both the methacrylate-thiol-allyl ether and methacrylate-thiol-norbornene systems exhibited dramatic reductions in shrinkage stress (1.1+/-0.1 and 1.1+/-0.2 MPa, respectively) relative to BisGMA/TEGDMA (2.6+/-0.2 MPa).The improved polymerization kinetics and overall functional group conversion, coupled with reductions in shrinkage stress while maintaining equivalent flexural modulus, result in a superior overall dental restorative material as compared to traditional bulk dimethacrylate resins.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
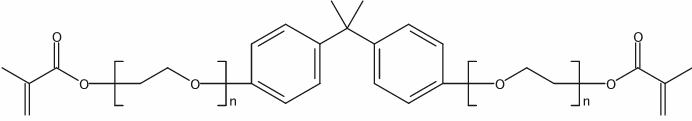 |
乙氧化双酚 A 甲基丙烯酸双酯
CAS:41637-38-1 |
(C2H4O)n(C2H4O)nC23H24O4 | |
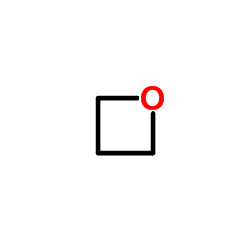 |
三羟甲基丙烷
CAS:77-99-6 |
C3H6O |
|
Antibacterial dental composites with chlorhexidine and mesop...
2014-12-01 [J. Dent. Res. 93(12) , 1283-9, (2014)] |
|
Effects of light exposure time on composite resin hardness a...
2008-07-01 [J. Dent. 36(7) , 520-8, (2008)] |
|
Novel F-releasing composite with improved mechanical propert...
2009-01-01 [J. Dent. Res. 88(1) , 83-8, (2009)] |
|
Tissue engineering scaffolds based on photocured dimethacryl...
2006-06-01 [Biomacromolecules 7(6) , 1751-7, (2006)] |
|
A comparison of the wear resistance and hardness of indirect...
2001-04-01 [J. Prosthet. Dent. 85(4) , 386-95, (2001)] |