| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
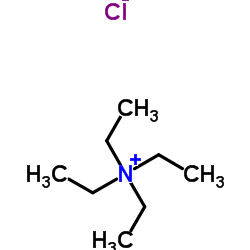 |
四乙基氯化铵
CAS:56-34-8 |
|
 |
甲醇
CAS:67-56-1 |
|
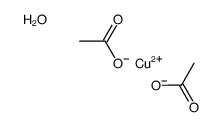 |
醋酸铜
CAS:6046-93-1 |
|
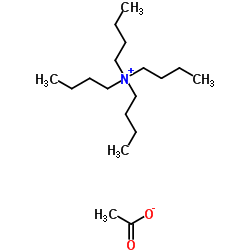 |
四丁基醋酸铵
CAS:10534-59-5 |
|
 |
乙酸铜盐水合物
CAS:66923-66-8 |