| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
D-苯基乳酸
CAS:7326-19-4 |
|
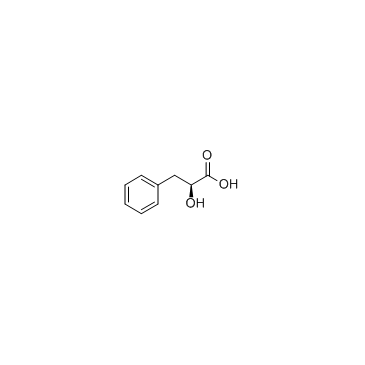 |
L-(-)-3-苯乳酸
CAS:20312-36-1 |
|
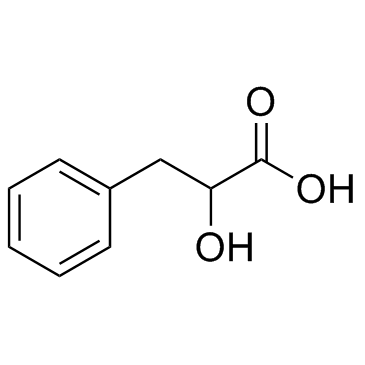 |
DL-3-苯基-2-羟丙酸
CAS:828-01-3 |