| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
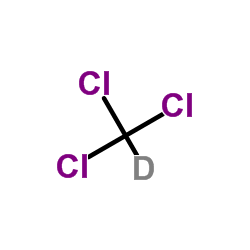 |
氘代氯仿-d
CAS:865-49-6 |
|
 |
伞形花内酯; 7-羟基香豆素
CAS:93-35-6 |
|
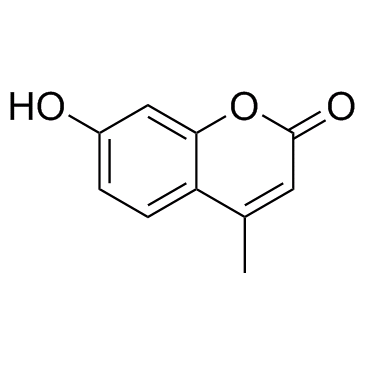 |
4-甲基伞形酮
CAS:90-33-5 |
|
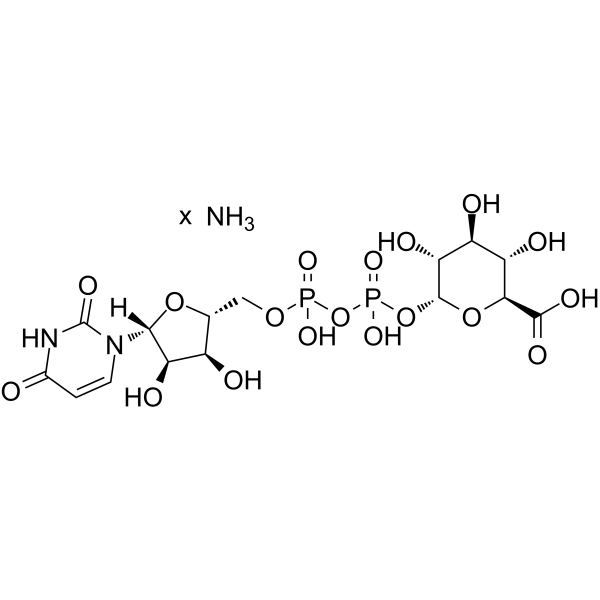 |
尿苷-5′-二磷酸葡糖醛酸 铵盐
CAS:43195-60-4 |
|
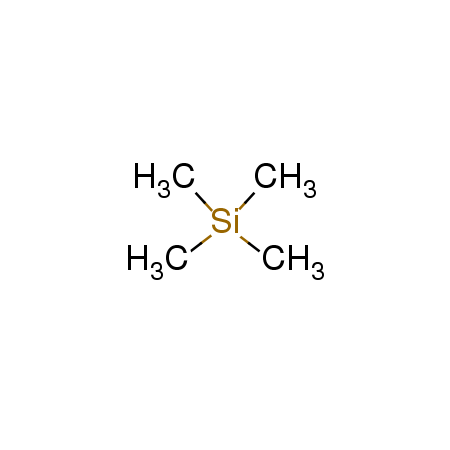 |
四甲基硅烷
CAS:75-76-3 |
|
 |
4-甲基-7-氧香豆素-β-D-吡喃半乳糖苷
CAS:6160-78-7 |