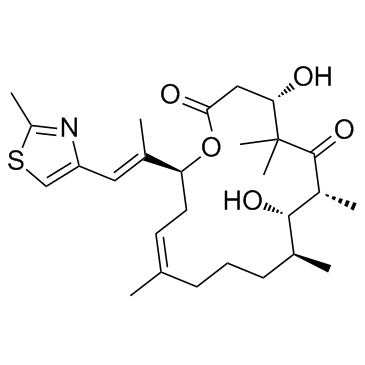
|
~99% |
|
~83% |
|
~92% |
|
~99% |
|
~87% |
|
~88% |
|
~% |
|
~% |
|
~70% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~94% |
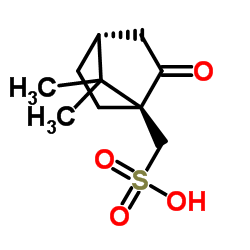
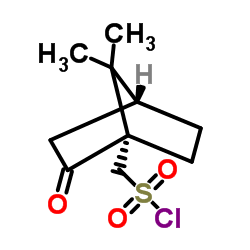

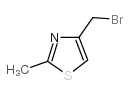


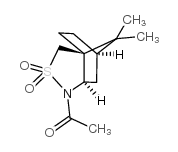
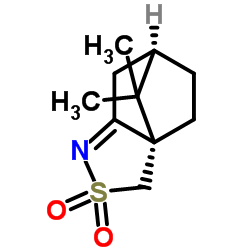
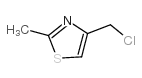
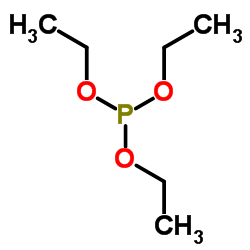
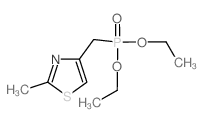
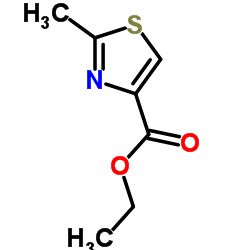
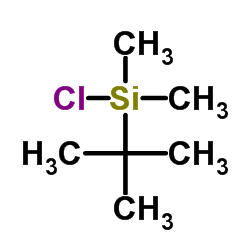
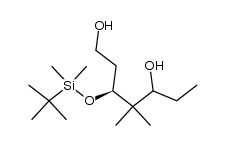
![(5S)-5-{[(tert-butyl)dimethylsilyl]oxy}-7-hydroxy-4,4-dimethylheptan-3-one结构式](https://image.chemsrc.com/caspic/004/250679-53-9.png)
![(5S)-5,7-Bis-{[tert-butyldimethylsilyl)oxy]}-4,4-dimethylheptan-3-one结构式](https://image.chemsrc.com/caspic/298/187527-25-9.png)

![(3S)-3-[((tert-butyl)dimethylsilyl)oxy]-1-[(1S,5R)-10,10-dimethyl-3,3-dioxido-3-thia-4-azatricyclo[5.2.1.01.5]dec-4-yl]-4,4-dimethylheptan-1,5-dione结构式](https://image.chemsrc.com/caspic/042/250679-52-8.png)
![(3S)-1-[(1S,5R)-10,10-dimethyl-3,3-dioxido-3-thia-4-azatricyclo[5.2.1.01.5]dec-4-yl]-3-hydroxy-4,4-dimethylheptan-1,5-dione结构式](https://image.chemsrc.com/caspic/080/250679-51-7.png)
![(13Z,4S,7R,8S,9S,16S)-4-(tert-butyldimethylsilyloxy)-8-hydroxy-5,5,7,9,13-pentamethyl-16-[(E)-1-methyl-2-(2-methylthiazol-4-yl)vinyl]oxacyclohexadec-13-ene-2,6-dione结构式](https://image.chemsrc.com/caspic/322/219823-99-1.png)