Validated LC-MS/MS assay for the quantitative determination of nalbuphine in human plasma and its application to a pharmacokinetic study.
Li-Jing Cai, Jun Zhang, Xiu-Mei Wang, R H Zhu, Jian Yang, Qi-Zhi Zhang, W X Peng
文献索引:Biomed. Chromatogr. 25(12) , 1308-14, (2011)
全文:HTML全文
摘要
A solid-phase extraction-liquid chromatographic-tandem mass spectrometry method for the determination of nalbuphine concentrations in human plasma has been developed. Samples (1 mL) were extracted using a Strata™-X solid phase extraction cartridges. Chromatographic separation of nalbuphine and naloxone (internal standard) was achieved on a Phenomenex Kinetex PFP (2.6 μm, 100 A, 100 × 2.1 mm) column using a mobile phase consisting of 0.1% formic acid, 15 mM ammonium acetate in deionized water and acetonitrile (60:40, v/v). The flow rate was 0.3 mL/min and the total run time was 2 min. Detection of the analytes was achieved using positive ion electrospray ionization via multiple reactions monitoring mode. The mass transitions were m/z 358 → 340 for nalbuphine and m/z 328 → 310 for naloxone. The assay was linear over the concentration range 0.50-500.00 ng/mL, with correlation coefficients ≥0.995. The lower limit of quantitation was set at 0.5 ng/mL plasma based on an average signal-to-noise ratio of 44.79. The intra- and inter-day precision was less than 8.07% in terms of relative standard deviation and accuracy ranged from 94.97 to 106.29% at all quality control levels. The method was applied successfully to determine nalbuphine concentrations in human plasma samples obtained from subjects receiving intravenous administration of nalbuphine. The method is rapid, sensitive, selective and directly applicable to human pharmacokinetic studies involving nalbuphine.Copyright © 2011 John Wiley & Sons, Ltd.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
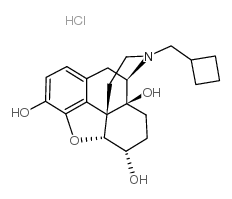 |
纳布啡盐酸盐水合物
CAS:23277-43-2 |
C21H28ClNO4 |
|
Pharmacokinetics of nalbuphine hydrochloride after intraveno...
2011-06-01 [Am. J. Vet. Res. 72(6) , 741-5, (2011)] |
|
A review of systemic opioids commonly used for labor pain re...
2011-01-01 [J. Midwifery Womens. Health 56(3) , 222-39, (2011)] |
|
Ultrasound guidance characteristics and efficiency of supraz...
2012-09-01 [Paediatr. Anaesth. 22(9) , 841-6, (2012)] |
|
Evaluation of topical nalbuphine or oral tramadol as analges...
2011-11-01 [Vet. Ophthalmol. 14(6) , 358-64, (2011)] |
|
Pain facilitation brain regions activated by nalbuphine are ...
2013-01-01 [PLoS ONE 8(1) , e50169, (2013)] |