| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
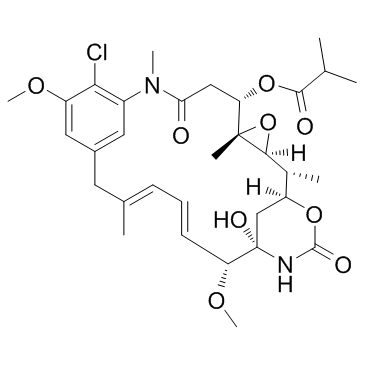 |
安丝菌素P3
CAS:66584-72-3 |
|
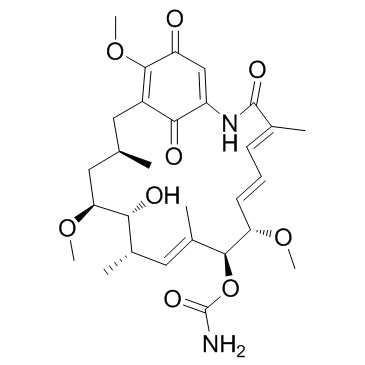 |
格尔德霉素
CAS:30562-34-6 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
安丝菌素P3
CAS:66584-72-3 |
|
 |
格尔德霉素
CAS:30562-34-6 |