Artemisinin antimalarials moderately affect cytochrome P450 enzyme activity in healthy subjects.
Sara Asimus, Doaa Elsherbiny, Trinh N Hai, Britt Jansson, Nguyen V Huong, Max G Petzold, Ulrika S H Simonsson, Michael Ashton
文献索引:Fundam. Clin. Pharmacol. 21(3) , 307-16, (2007)
全文:HTML全文
摘要
The aim of this study was to investigate which principal human cytochrome P450 (CYP450) enzymes are affected by artemisinin and to what degree the artemisinin derivatives differ with respect to their respective induction and inhibition capacity. Seventy-five healthy adults were randomized to receive therapeutic oral doses of artemisinin, dihydroartemisinin, arteether, artemether or artesunate for 5 days (days 1-5). A six-drug cocktail consisting of caffeine, coumarin, mephenytoin, metoprolol, chlorzoxazone and midazolam was administered orally on days -6, 1, 5 and 10 to assess the activities of CYP1A2, CYP2A6, CYP2C19, CYP2D6, CYP2E1 and CYP3A, respectively. Four-hour plasma concentrations of parent drugs and corresponding metabolites and 7-hydroxycoumarin urine concentrations were quantified by liquid chromatography-tandem mass spectrometry. The 1-hydroxymidazolam/midazolam 4-h plasma concentration ratio (CYP3A) was increased on day 5 by artemisinin [2.66-fold (98.75% CI: 2.10-3.36)], artemether [1.54 (1.14-2.09)] and dihydroartemisinin [1.25 (1.06-1.47)] compared with day -6. The S-4'-hydroxymephenytoin/S-mephenytoin ratio (CYP2C19) was increased on day 5 by artemisinin [1.69 (1.47-1.94)] and arteether [1.33 (1.15-1.55)] compared with day -6. The paraxanthine/caffeine ratio (CYP1A2) was decreased on day 1 after administration of artemisinin [0.27 (0.18-0.39)], arteether [0.70 (0.55-0.89)] and dihydroartemisinin [0.73 (0.59-0.90)] compared with day -6. The alpha-hydroxymetoprolol/metoprolol ratio (CYP2D6) was lower on day 1 compared with day -6 in the artemisinin [0.82 (0.70-0.96)] and dihydroartemisinin [0.83 (0.71-0.96)] groups, respectively. In the artemisinin-treated subjects this decrease was followed by a 1.34-fold (1.14-1.58) increase from day 1 to day 5. These results show that intake of artemisinin antimalarials affect the activities of several principal human drug metabolizing CYP450 enzymes. Even though not significant in all treatment groups, changes in the individual metrics were of the same direction for all the artemisinin drugs, suggesting a class effect that needs to be considered in the development of new artemisinin derivatives and combination treatments of malaria.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
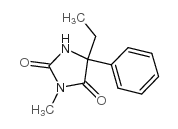 |
美芬妥因
CAS:50-12-4 |
C12H14N2O2 |
|
Cheminformatics analysis of assertions mined from literature...
2010-01-01 [Chem. Res. Toxicol. 23 , 171-83, (2010)] |
|
Translating clinical findings into knowledge in drug safety ...
2011-12-01 [J. Sci. Ind. Res. 65(10) , 808, (2006)] |
|
Multi-target spectral moment QSAR versus ANN for antiparasit...
2010-03-15 [Bioorg. Med. Chem. 18 , 2225-31, (2010)] |
|
Quantitative structure-activity relationship and complex net...
2008-11-13 [J. Med. Chem. 51 , 6740-51, (2008)] |
|
Assessment of urinary mephenytoin metrics to phenotype for C...
2008-04-01 [Eur. J. Clin. Pharmacol. 64(4) , 387-98, (2008)] |