| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
氯化钠
CAS:7647-14-5 |
|
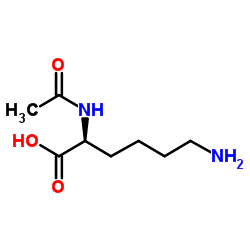 |
Nα-乙酰-L-赖氨酸
CAS:1946-82-3 |
|
 |
4-羟乙基哌嗪乙磺酸
CAS:7365-45-9 |
|
 |
氯化钠-35cl
CAS:20510-55-8 |
|
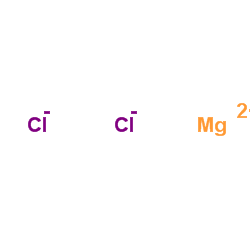 |
氯化镁
CAS:7786-30-3 |
|
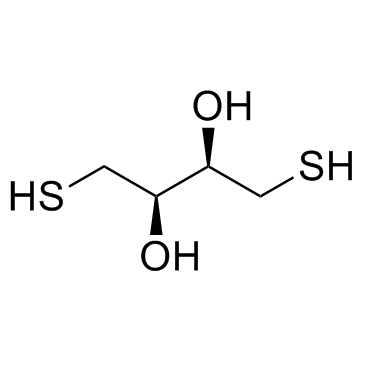 |
DL-二硫苏糖醇
CAS:3483-12-3 |
|
 |
乙二胺四乙酸
CAS:60-00-4 |