| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
氯化钠
CAS:7647-14-5 |
|
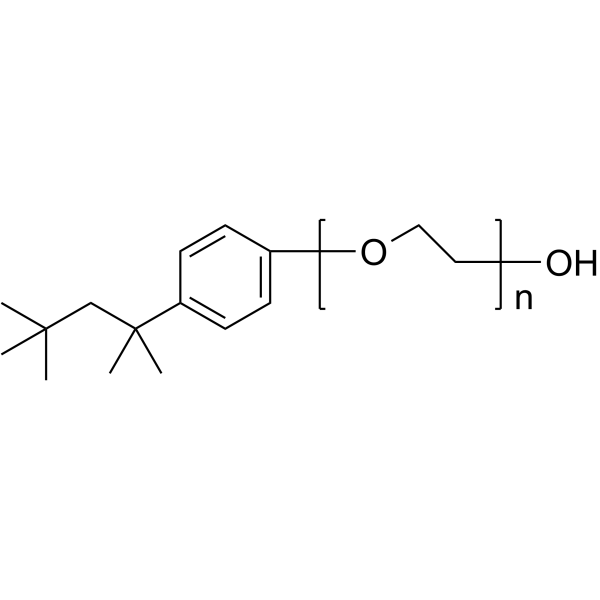 |
曲拉通X-100
CAS:9002-93-1 |
|
 |
氯化钠-35cl
CAS:20510-55-8 |
|
 |
乙二胺四乙酸
CAS:60-00-4 |
|
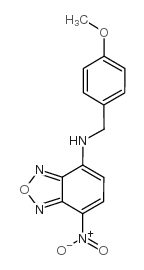 |
7-(对甲氧基苄氨基)-4-硝基苯-2-恶唑-1,3-二唑
CAS:33984-50-8 |