| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
氯化钠
CAS:7647-14-5 |
|
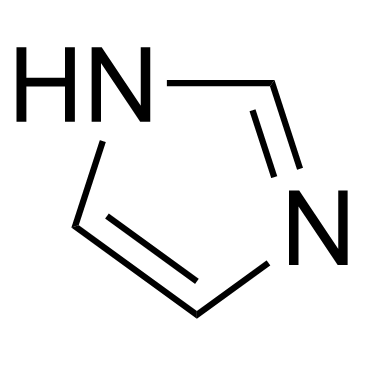 |
咪唑
CAS:288-32-4 |
|
 |
氯化钠-35cl
CAS:20510-55-8 |
|
 |
大麻二酚
CAS:13956-29-1 |