Solid-phase synthesis of a range of O-phosphorylated peptides by post-assembly phosphitylation and oxidation.
D M Andrews, J Kitchin, P W Seale
文献索引:Int. J. Pept. Protein Res. 38(5) , 469-75, (1991)
全文:HTML全文
摘要
A completely general method for the O-phosphorylation of peptides of any given composition using solid-phase methodology is described. Peptides were assembled using Fmoc amino acid active esters, with base used for Fmoc deprotection. Unprotected amino acid side chain hydroxyl groups were phosphitylated and oxidised at the end of the assembly using bis(benzyloxy)(diisopropylamino)phosphine and tert.-butylhydroperoxide respectively. TFA was used for final deprotection of the amino acid side chains and for simultaneous cleavage from the resin. The synthesis of O-phosphopeptides of up to 15 residues in length is described.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
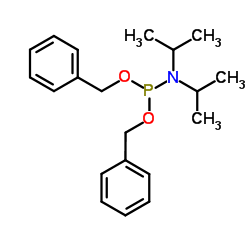 |
二苄基 N,N-二异丙基亚磷酰胺
CAS:108549-23-1 |
C20H28NO2P |
|
Therapeutic targeting of oncogenic K-Ras by a covalent catal...
2014-01-03 [Angew. Chem. Int. Ed. Engl. 53(1) , 199-204, (2014)] |
|
Differential hormone-dependent phosphorylation of progestero...
2000-01-01 [Mol. Endocrinol. 14(1) , 52-65, (2000)] |
|
A new reaction for the direct conversion of 4-azido-4-deoxy-...
2004-04-29 [Org. Lett. 6 , 1365-1368, (2004)] |
|
Polyphosphates and pyrophosphates of hexopyranoses as allost...
2011-01-03 [ChemMedChem 6(1) , 153-68, (2011)] |
|
[Synthesis 9 , 1481-1485, (2004)] |