NAD (P) H-dependent reduction of nicotinamide N-oxide by an unique enzyme system consisting of liver microsomal NADPH-cytochrome C reductase and cytosolic aldehyde oxidase.
S Kitamura, Y Wada, K Tatsumi
文献索引:Biochem. Biophys. Res. Commun. 125(3) , 1117-22, (1984)
全文:HTML全文
摘要
NAD (P) H-dependent reduction of nicotinamide N-oxide was investigated with rabbit liver preparations. Microsomes, microsomal NADPH-cytochrome c reductase or cytosolic aldehyde oxidase alone exhibited no nicotinamide N-oxide reductase activity in the presence of NADPH or NADH. However, when the microsomal preparations were combined with the cytosolic enzyme, a significant N-oxide reductase activity was observed in the presence of the reduced pyridine nucleotide. The activity was enhanced by FAD or methyl viologen. Cytosol alone supplemented with NADPH or NADH exhibited only a slight, but when combined with microsomes, a significant N-oxide reductase activity. Based on these facts, we propose a new electron transfer system consisting of NADPH-cytochrome c reductase and aldehyde oxidase, which exhibits nicotinamide N-oxide reductase activity in the presence of the reduced pyridine nucleotide.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
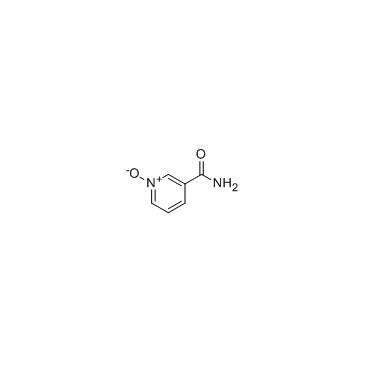 |
烟碱-N-氧化物
CAS:1986-81-8 |
C6H6N2O2 |
|
Effects of excess nicotinamide administration on the urinary...
2004-01-01 [Biosci. Biotechnol. Biochem. 68(1) , 44-50, (2004)] |
|
Pharmacokinetics and biochemistry studies on nicotinamide in...
1994-01-01 [Cancer Chemother. Pharmacol. 34(5) , 399-404, (1994)] |
|
Plasma and urine pharmacokinetics of niacin and its metaboli...
2007-08-01 [Int. J. Clin. Pharmacol. Ther. 45(8) , 448-54, (2007)] |
|
Niacin catabolism in rodents.
1990-04-01 [J. Nutr. Sci. Vitaminol. 36(2) , 87-98, (1990)] |
|
Can a 1,2-oxygen shift of a nicotinamide N-oxide derivative ...
1985-10-01 [Xenobiotica 15(10) , 799-803, (1985)] |