| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
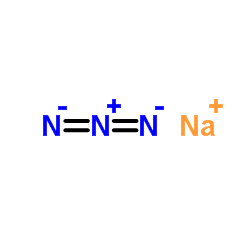 |
叠氮化钠
CAS:26628-22-8 |
|
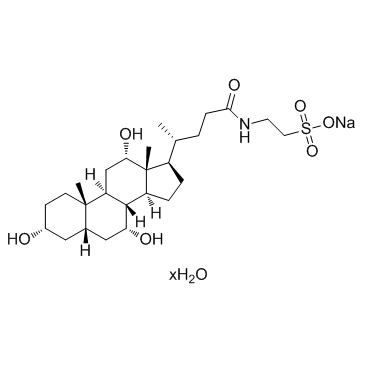 |
牛磺胆酸钠盐 水合
CAS:345909-26-4 |
|
 |
呋噻米
CAS:54-31-9 |
|
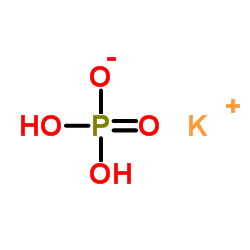 |
磷酸二氢钾
CAS:7778-77-0 |