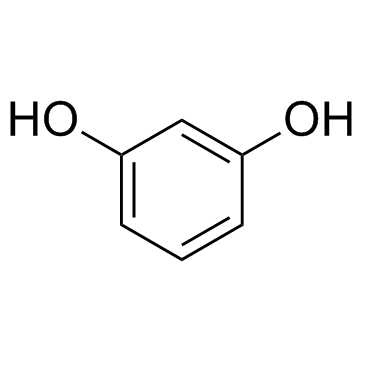
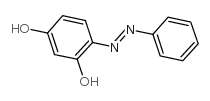
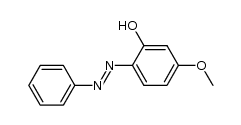
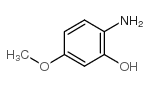
|
~% |
|
~92% |
|
~87% |
|
~81% |
|
~72% |
|
~% |
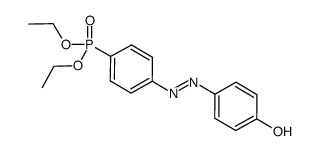
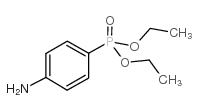
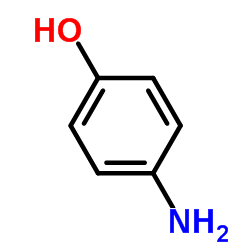
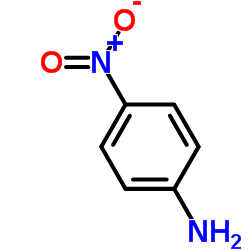
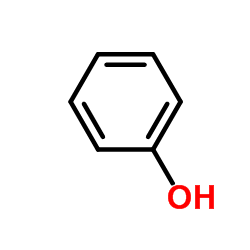
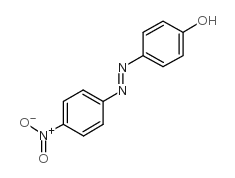
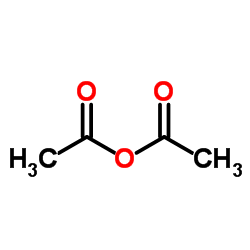
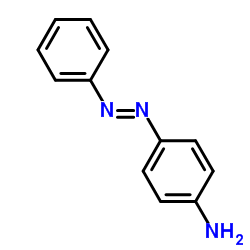
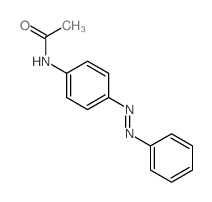
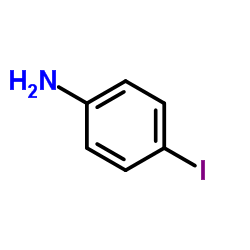
![Phenol,4-[2-(4-iodophenyl)diazenyl]结构式](https://image.chemsrc.com/caspic/374/2703-28-8.png)