| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
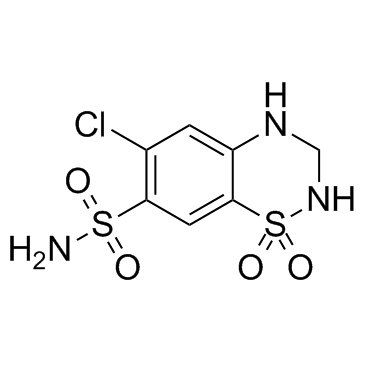 |
氢氯噻嗪
CAS:58-93-5 |
|
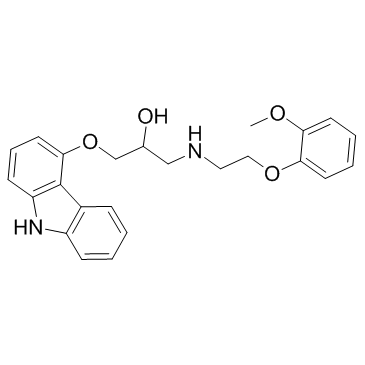 |
卡维地洛
CAS:72956-09-3 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
氢氯噻嗪
CAS:58-93-5 |
|
 |
卡维地洛
CAS:72956-09-3 |