| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
氯仿
CAS:67-66-3 |
|
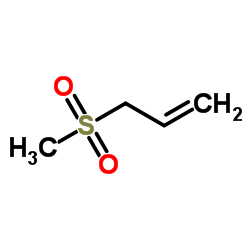 |
烯丙基甲基砜
CAS:16215-14-8 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
氯仿
CAS:67-66-3 |
|
 |
烯丙基甲基砜
CAS:16215-14-8 |