Novel thiazole-based heterocycles as selective inhibitors of fibrinogen-mediated platelet aggregation.
P J Sanfilippo, M J Urbanski, K N Beers, A Eckardt, R Falotico, M H Ginsberg, S Offord, J B Press, J Tighe, K Tomko
文献索引:J. Med. Chem. 38 , 34, (1995)
全文:HTML全文
摘要
The synthesis and biological activity of novel thiazole-based heterocycles as inhibitors of thrombin-induced human platelet aggregation are described. Further evaluation of selected compounds show they inhibit platelet aggregation as stimulated by a variety of agonists. The more active compounds also were found to inhibit fibrinogen binding to platelets. To further delineate the mechanism of action of these compounds, direct binding studies with the purified glycoprotein (GP) IIb/IIIa receptor were performed. Flow cytometry analyses of 24 and 32 indicate that these compounds block the activation process of the GPIIb/IIIa receptor without denaturing the integrin receptor. On the basis of these studies, 32 exhibited the best profile as a novel nonpeptide inhibitor of fibrinogen-mediated platelet aggregation.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
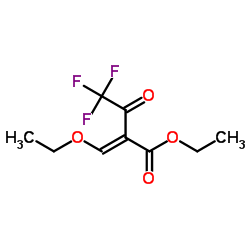 |
2-(乙氧基亚甲基)-4,4,4-三氟-3-氧代丁酸乙酯
CAS:571-55-1 |
C9H11F3O4 |
|
High-speed microwave-assisted synthesis of the trifluorometh...
2009-11-01 [ChemMedChem 4(11) , 1816-8, (2009)] |
|
Comparisons of pKa and log P values of some carboxylic and p...
2001-01-01 [AAPS PharmSci 3(2) , E10, (2001)] |
|
Synthesis and cardiotonic activity of 2-substituted 5-cyano-...
1992-04-01 [Il Farmaco 47 , 427, (1992)] |
|
Synthesis, antiviral (HSV-1) and antimycotic activities of e...
1993-03-01 [Il Farmaco 48 , 335, (1993)] |
|
[Eur. J. Med. Chem. 28 , 853, (1993)] |