| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
甲酸
CAS:64-18-6 |
|
 |
乙腈
CAS:75-05-8 |
|
 |
甲醇
CAS:67-56-1 |
|
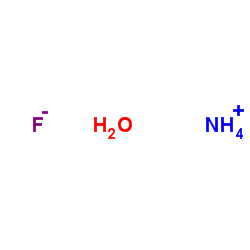 |
氟化铵
CAS:12125-01-8 |
|
 |
二甲基亚砜
CAS:67-68-5 |
|
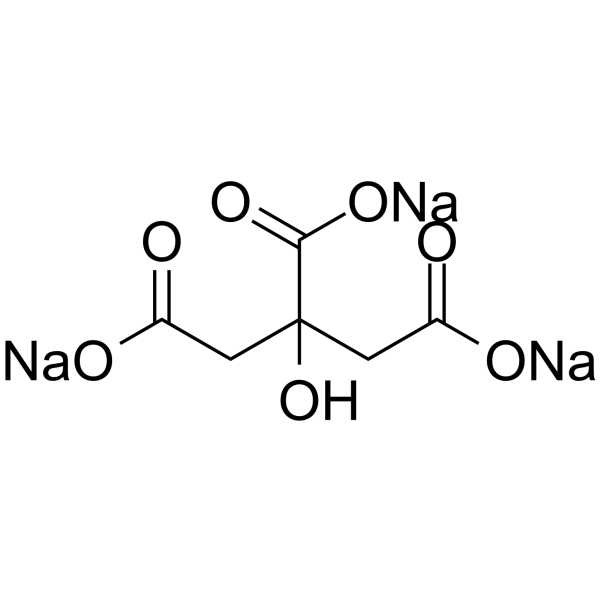 |
柠檬酸钠
CAS:68-04-2 |
|
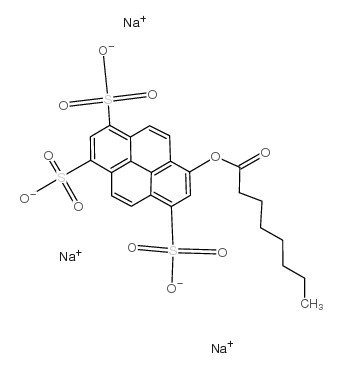 |
8-辛酰氧基芘-1,3,6-三磺酸三钠盐
CAS:115787-84-3 |