| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
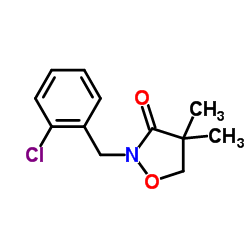 |
异恶草酮
CAS:81777-89-1 |
|
 |
过硫酸钠
CAS:7775-27-1 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
异恶草酮
CAS:81777-89-1 |
|
 |
过硫酸钠
CAS:7775-27-1 |