| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
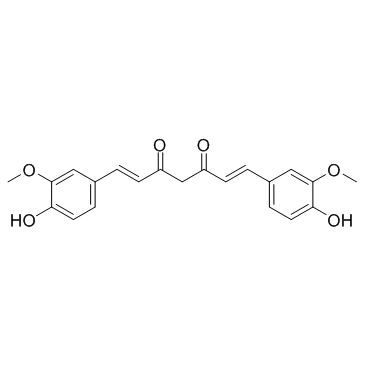 |
姜黄素
CAS:458-37-7 |
|
 |
氯化钠
CAS:7647-14-5 |
|
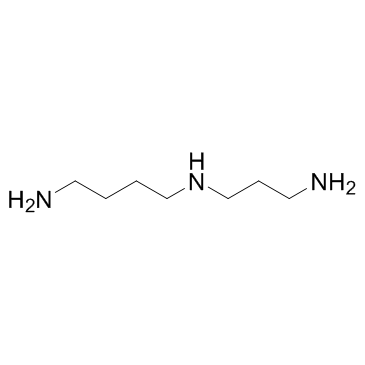 |
亚精胺
CAS:124-20-9 |
|
 |
十二烷基硫酸钠
CAS:151-21-3 |
|
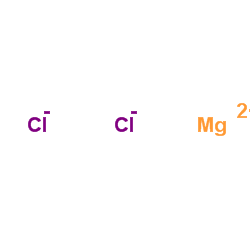 |
氯化镁
CAS:7786-30-3 |
|
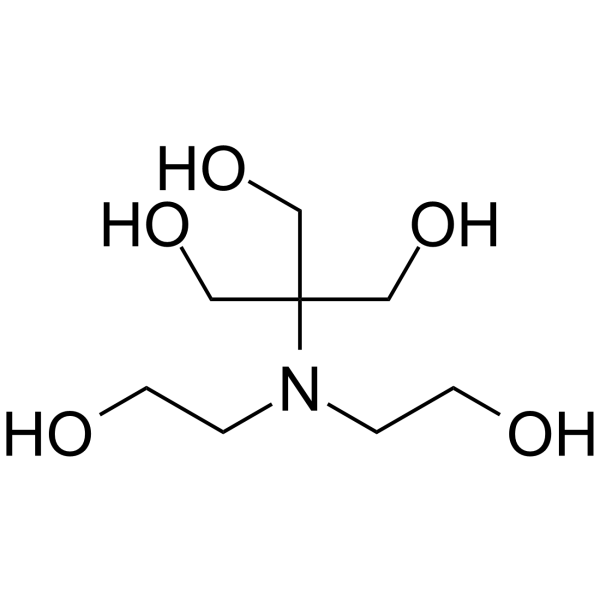 |
二(2-羟乙基)亚氨基三(羟甲基)甲烷
CAS:6976-37-0 |
|
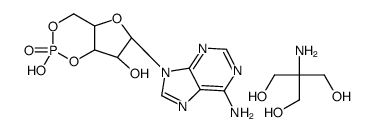 |
腺苷3',5'-环单磷酸三羟甲基氨基甲烷盐
CAS:102029-77-6 |
|
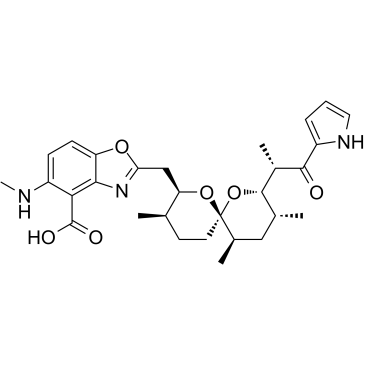 |
钙离子载体 III
CAS:52665-69-7 |
|
 |
氯化钠-35cl
CAS:20510-55-8 |
|
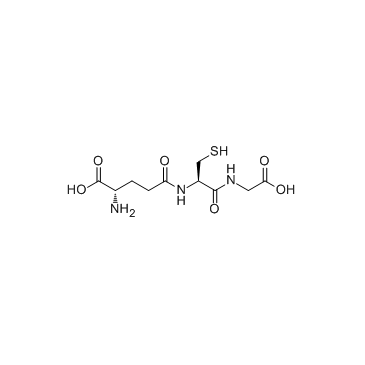 |
谷胱甘肽/5-L-谷氨酰-L-半胱氨酰甘氨酸
CAS:70-18-8 |