Simultaneous determination of ezetimibe and simvastatin in pharmaceutical preparations by MEKC.
Ceren Yardimci, Nuran Ozaltin
文献索引:J. Chromatogr. Sci. 48(2) , 95-9, (2010)
全文:HTML全文
摘要
A micellar electrokinetic capillary chromatography method was developed and validated for the simultaneous determination of ezetimibe and simvastatin in pharmaceutical preparations. The influence of buffer concentration, buffer pH, sodium dodecyl sulphate (SDS) concentration, organic modifier, capillary temperature, applied voltage, and injection time was investigated, and the method validation studies were performed. The optimum separation for these analytes was achieved in less than 10 min at 30 degrees C with a fused-silica capillary column (56 cm x 50 microm i.d.) and a 25mM borate buffer at pH 9.0 containing 25mM SDS and 10% (v/v) acetonitrile. The samples were injected hydrodynamically for 3 s at 50 mbar, and the applied voltage was +30.0 kV. Detection wavelength was set at 238 nm. Diflunisal was used as internal standard. The method was suitably validated with respect to stability, specificity, linearity, limits of detection and quantification, accuracy, precision, and robustness. The limits of detection and quantification were 1.0 and 2.0 microg/mL for both ezetimibe and simvastatin, respectively. The method developed was successfully applied to the simultaneous determination of ezetimibe and simvastatin in pharmaceutical preparations.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
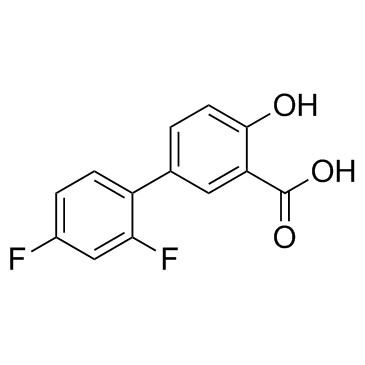 |
二氟尼柳
CAS:22494-42-4 |
C13H8F2O3 |
|
Synthesis and biological evaluation of amide derivatives of ...
2009-08-01 [Bioorg. Med. Chem. Lett. 19(15) , 4399-402, (2009)] |
|
Evaluation of the drug-polymer interaction in calcium algina...
2010-02-01 [Pharmazie 65(2) , 106-9, (2010)] |
|
Simultaneous determination of the two non-steroidal anti-inf...
2009-01-01 [Pak. J. Pharm. Sci. 22(1) , 8-17, (2009)] |
|
Cyclodextrin-crosslinked poly(acrylic acid): adhesion and co...
2013-03-01 [AAPS PharmSciTech 14(1) , 301-11, (2013)] |
|
A new hydrate form of diflunisal precipitated from a microem...
2013-09-01 [Colloids Surf. B Biointerfaces 109 , 68-73, (2013)] |