| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
4-甲基-2-氧戊酸
CAS:816-66-0 |
|
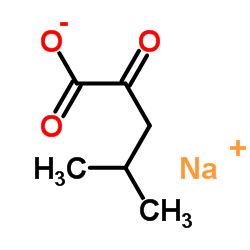 |
4-甲基-2-氧代戊酸钠盐
CAS:4502-00-5 |