| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
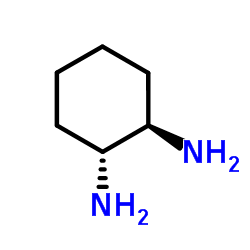 |
反式-1,2-环己二胺
CAS:1121-22-8 |
|
 |
顺-1,2-环己二胺
CAS:1436-59-5 |
|
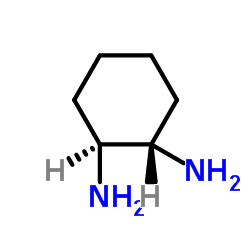 |
(1R,2R)-(-)-1,2-环己二胺
CAS:20439-47-8 |
|
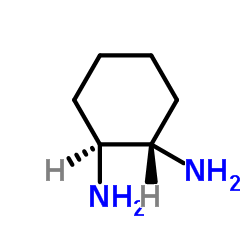 |
(1S,2S)-(+)-1,2-环己二胺
CAS:21436-03-3 |
|
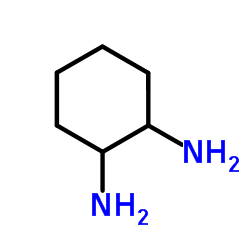 |
1,2-环己二胺
CAS:694-83-7 |