| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
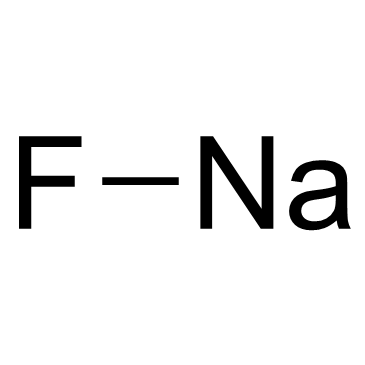 |
氟化钠
CAS:7681-49-4 |
|
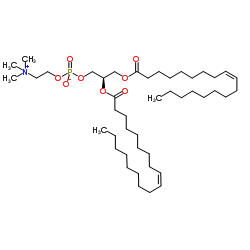 |
1,2-二油酰基-sn-甘油-3-磷酸胆
CAS:4235-95-4 |
|
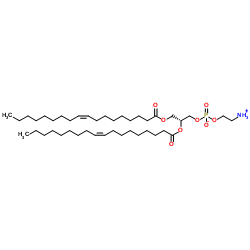 |
1,2-二油酰基-sn-丙三基-3-磷脂酰乙醇胺
CAS:4004-05-1 |
|
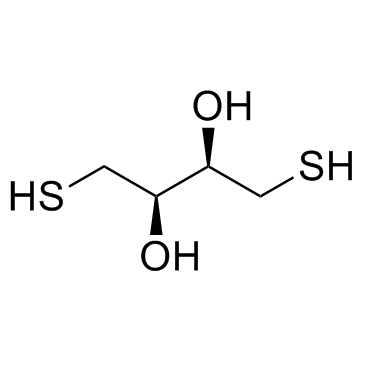 |
DL-二硫苏糖醇
CAS:3483-12-3 |