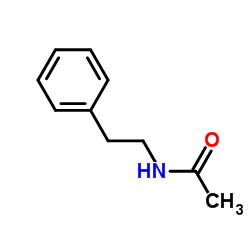
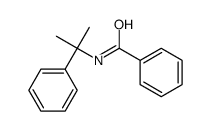
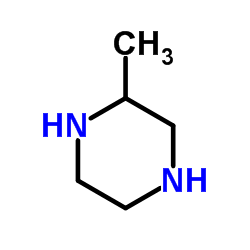
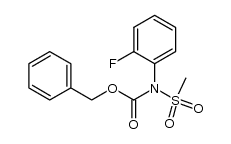
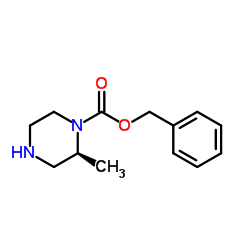
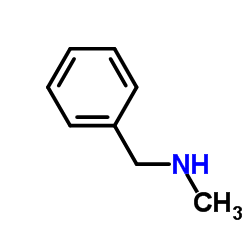
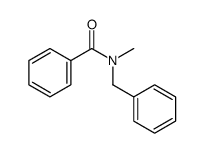
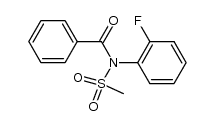
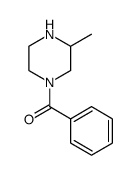
|
~% |
|
~% |
|
~92% |
|
~73% |
|
~% |
|
~76% |
|
~86% |
|
~% |
|
~94% |
|
~94% |
|
~% |
|
~90% |
|
~87% |
|
~96% |
|
~% |
|
~91% |
|
~90% |
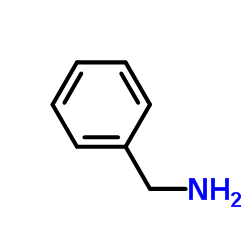

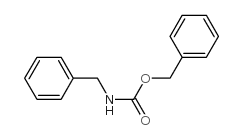
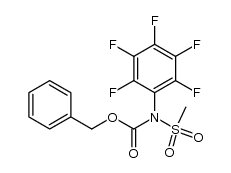

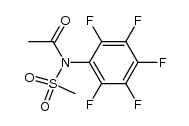
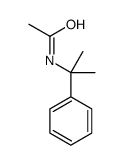
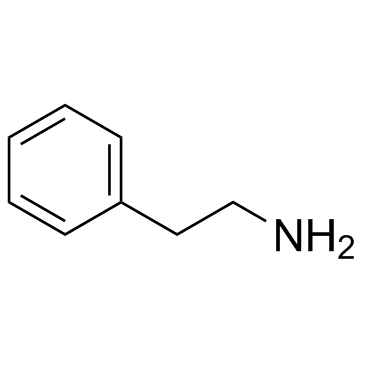
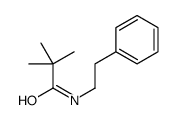
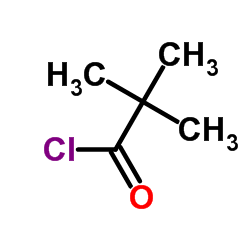
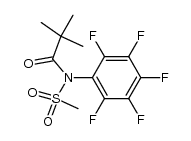
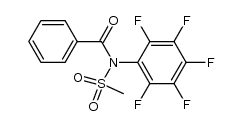
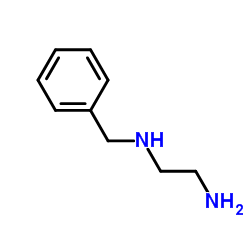
![N-[2-(benzylamino)ethyl]benzamide结构式](https://image.chemsrc.com/caspic/055/67630-76-6.png)