| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
硫酸
CAS:7664-93-9 |
|
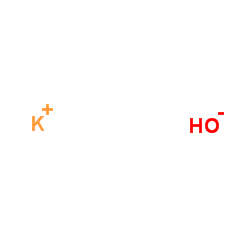 |
氢氧化钾
CAS:1310-58-3 |
|
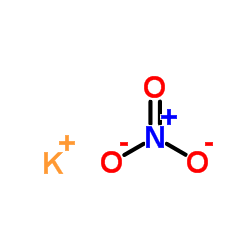 |
硝酸钾
CAS:7757-79-1 |
|
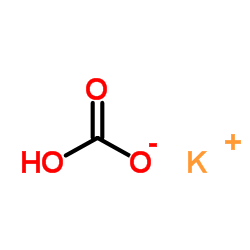 |
碳酸氢钾
CAS:298-14-6 |
|
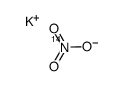 |
potassium,dioxido(oxo)azanium
CAS:1173018-94-4 |