| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
甘油
CAS:56-81-5 |
|
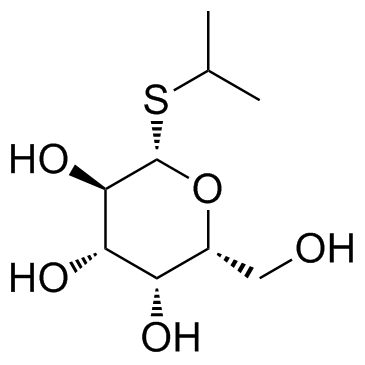 |
异丙基-β-D-硫代半乳糖苷(IPTG)
CAS:367-93-1 |
|
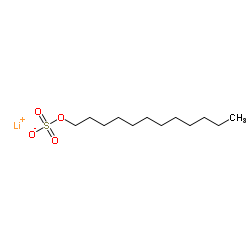 |
十二烷基硫酸锂
CAS:2044-56-6 |
|
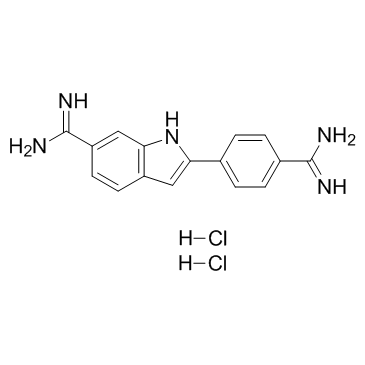 |
4',6-二脒基-2-苯基吲哚二盐酸盐
CAS:28718-90-3 |
|
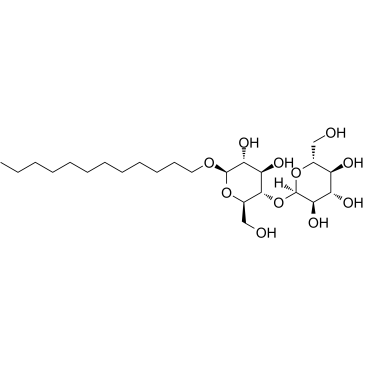 |
十二烷基-beta-D-麦芽糖苷
CAS:69227-93-6 |
|
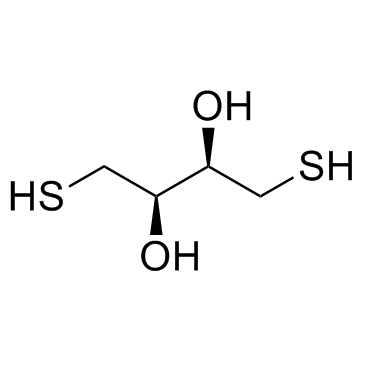 |
DL-二硫苏糖醇
CAS:3483-12-3 |
|
![N-[1-(2,3-二油酰氧基)丙基]-N,N,N-三甲基铵甲基-硫酸盐 结构式](https://image.chemsrc.com/caspic/484/144189-73-1.png) |
N-[1-(2,3-二油酰氧基)丙基]-N,N,N-三甲基铵甲基-硫酸盐
CAS:144189-73-1 |