|
~% |
|
~% |
|
~59% |
|
~% |
|
~% |
|
~80% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
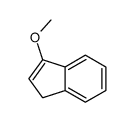
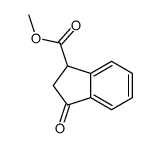




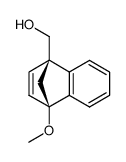
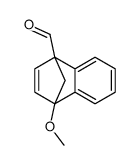

![methyl (3aR,4R,9S,9aS)-9-methoxy-1,3-dioxo-3,3a,9,9a-tetrahydro-4,9-methanonaphtho[2,3-c]furan-4(1H)-carboxylate结构式](https://image.chemsrc.com/caspic/140/91948-51-5.png)