|
~93% |
|
~87% |
|
~% |
|
~48% |
|
~% |
|
~32% |
|
~% |

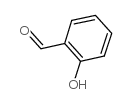
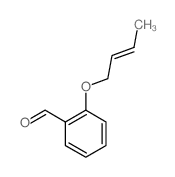
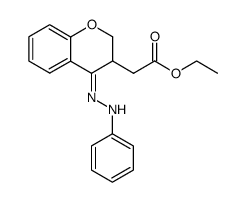
![2-phenyl-4a,5-dihydro-4H-chromeno[4,3-c]pyridazin-3-one结构式](https://image.chemsrc.com/caspic/414/74553-37-0.png)