|
~% |
|
~% |
|
~% |
|
~% |
|
~99% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
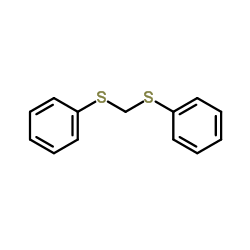
![[1-(benzenesulfonyl)cyclobutyl]sulfonylbenzene结构式](https://image.chemsrc.com/caspic/324/28246-89-1.png)

![[1-(benzenesulfonyl)cyclopentyl]sulfonylbenzene结构式](https://image.chemsrc.com/caspic/450/88073-51-2.png)

![[1-(benzenesulfonyl)-3-chloropropyl]sulfonylbenzene结构式](https://image.chemsrc.com/caspic/234/89593-83-9.png)
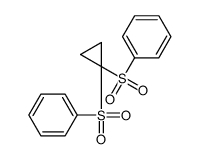
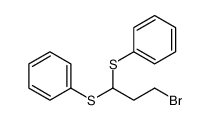
![[1-(benzenesulfonyl)-3-iodopropyl]sulfonylbenzene结构式](https://image.chemsrc.com/caspic/186/89593-84-0.png)

![[1-(benzenesulfonyl)-5-chloropentyl]sulfonylbenzene结构式](https://image.chemsrc.com/caspic/072/89593-87-3.png)
![[1-(benzenesulfonyl)-5-iodopentyl]sulfonylbenzene结构式](https://image.chemsrc.com/caspic/034/89593-88-4.png)